Schrödinger's equation - a journey of physics simulation
DISCLAIMER: Yes, we know the difference between
Schrodinger and Schrödinger. But, for
simplicity (and typing speed), they will be used interchangably. I
aplologize upfront.
This project is not finished yet. And it is definitely not yet compliant with realistic physics standards. At least, we tried.
Majorly misunderstood explaination
According to a pretty modern theory, quantum mechanics, all matter in
the universe is composed of tiny particles, the basic
building blocks of the world. Now, we have something like a "periodic
table" of particles. The standard model.
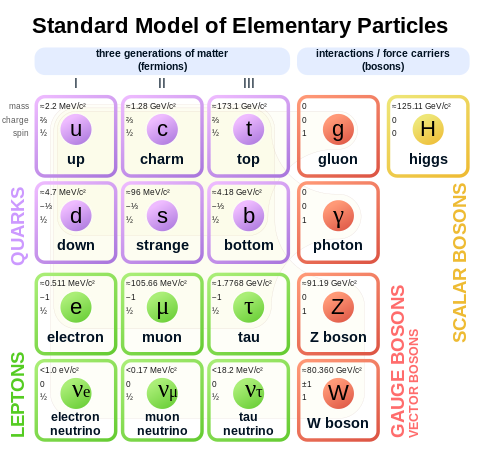
But here's the thing. Particles are so small that we can't really measure where they are. And on this scale, when the particle size is noting compared to the uncertainty of its position (or momentum, for that matter), we cannot even speak of a certain "location" in space and time. And we can't just take better measurments in order to decrease the uncertainty. Because we have both position and momentum uncertainty. And measuring one of them will inevitably interfere with the other. So much that there is a special equation - the Heisenberg Uncertainty principle, linking those values together.

- σ_x is the uncertainty of position
- σ_p is the uncertainty of momentum
-
ℏ is the reduced Planck constant,
1.05457148 × 10^-34 m^2 kg / s
If there is no certain location, then what is there? The naive answer would be: propability. But propability requires a finite number of possible states or values - eigenstates. Here, we have an uncountable infinity of them. We need an infinitely small propability for every one of the infinite points in space. And assigning a single propability to each point implies... a function. The wave function is an object closest to the position of a subatomic particle.
Properties of the wave function
The wave function assigns a complex number to each point in space and time. The square of this value is the propability density. It is a finite number, not infinitely small. The propability of the particle existing in a given range of space is a definite integral of the square of the absolute value of the wave function.
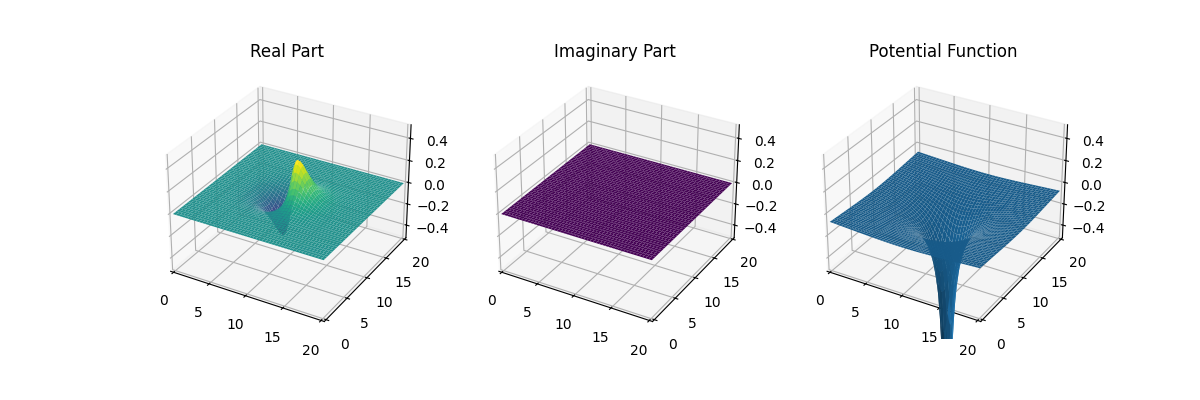
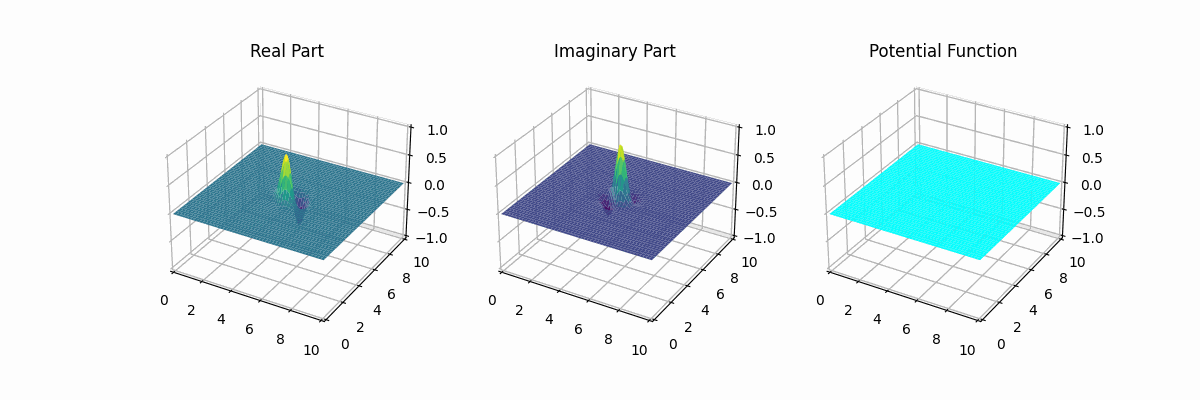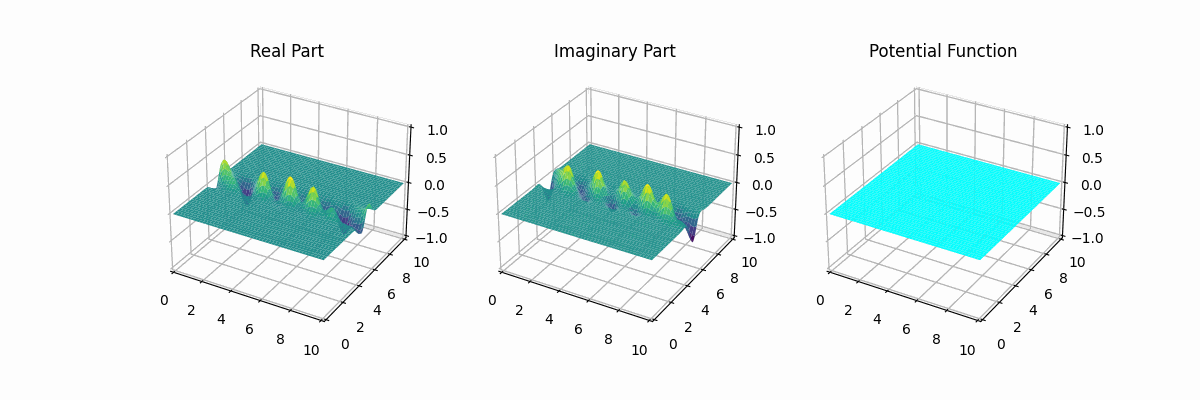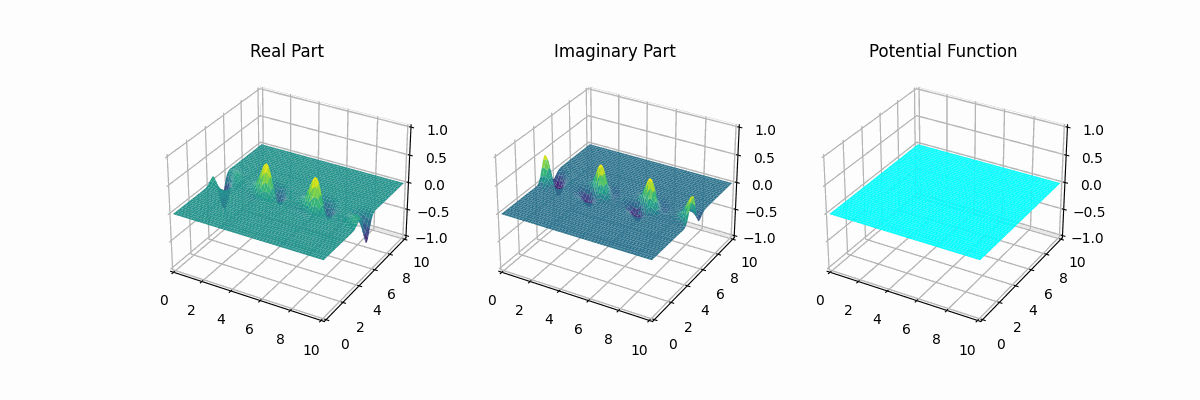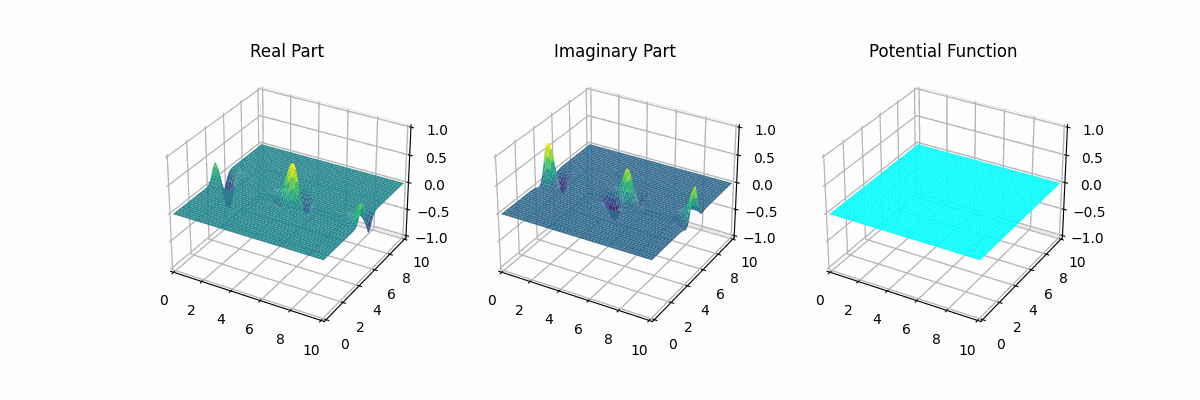
I made the graph myself...
This is a typical wave-function-graph of an electron in a hydrogen atom
at ground level. It is pretty straightforward that there is a higher
chance of finding an electron where the square of the absolute value
(Re^2 + Im^2) is higher. The real and imaginary part are
separate, just for clarity. Later they will not always be (we will show
phase and absolute).
The potential function is another abstract concept. It is a value for the particle to interact with external forces and particles. Particles are atracted to places with a potential lower than their wave function, and repeled from places where their wave function is lower than the potential around them.
Evolution in time

Propably Erwin Schrödinger too
This is the Schrödinger's equation. It describes the evolution in time
of the wave function (𝛙) based on the potential (V). We are solving it
using scipy and numpy for arbitrary functions,
some of which supplied by the user. That way we get an accurate
representation of how particles move. Actually respecting quantum
physics.
I made the graph myself too...
Editor's note:
Stop before I start copyrighting these gradients
Limitations
1. Relativity
Our library simulates only non-relativistic quantum physics. And
the only reason for that is... computation power. Not everyone has a
computer capable of solving complex differential equations,
while accounting for spacetime curvature and doing Lorentz
transformations
2. Quantum field theory
We understand that when dealing with wave functions, there is no need to
count individual particles, since they should be just written down as a
single electron field, single photon field, and interacting with
superposed quasi-particles. We understand that electrons either repel
each other electromagnetically or emit photons. But here, we just
focused on visualising all the quantum phenomena in a simple way,
instead of implementing the tiniest and newest releases in difficult
physics papers.
yeah im really smart huh
3. Quantum chromodynamics
My complete misunderstanding of quarks and hadrons, and color charge
would leave even the wildest of physics-ignorants amazed by my scale of
stupidity. (only
mine,
KacperTZSTI
and
ErexPL
did just fine). But our main focus was computationally-efficient
kinda-realistic visualisations of most quantum phenomena, not complete
realism. If you need more realism, install a quantum physics Micro$oft
Minecraft ®© texture pack.